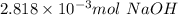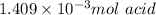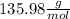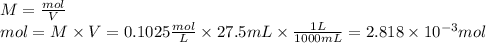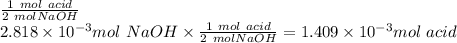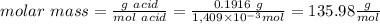Chemistry, 15.01.2020 19:31 eyeneedalife
1) a student dissolved 0.1916 g of an unknown diprotic acid in 100 ml of distilled water. the acid was then titrated with 0.1025m naoh solution. the second equivalence point showed the sharpest change in ph, and so it was used to determine the molar mass of the unknown acid. the volume of naoh needed to reach the equivalence point was 27.5 ml.
a. calculate the number of moles of naoh used in the titration to reach the second equivalence point.
b. calculate the number of moles of diprotic acid, based on the fact that we are examining the second equivalence point.
c. calculate the molar mass of the diprotic acid.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, portedon8644
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

Chemistry, 22.06.2019 02:30, carsonjohnsonn
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1


Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1
You know the right answer?
1) a student dissolved 0.1916 g of an unknown diprotic acid in 100 ml of distilled water. the acid w...
Questions in other subjects:





Mathematics, 01.06.2021 03:10

Mathematics, 01.06.2021 03:10