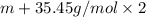Chemistry, 15.01.2020 19:31 whitakers87
An element x has a dibrom ide with the empirical formula xbr2 and a dichloride with the empirical formula xci2. the dibromide is completely converted to the dichloride when it is heated in a stream of chlorine according to the reaction $$xbr2 + cl2 → xci2 + br2$$ when 1.500 g xbr2 is treated, 0.890 g xci2 results. (a) calculate the atomic mass of the element x. (b) by reference to a list of the atomic masses of the elements, identify the element x.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, RedDemon59
Apeak with a retention time of 407 s has a width at half-height (w1/2) of 7.6 s. a neighboring peak is eluted 17 s later with a w1/2 of 9.4 s. a compound that is known not to be retained was eluted in 2.5 s. the peaks are not baseline resolved. how many theoretical plates would be needed to achieve a resolution of 1.5?
Answers: 2



Chemistry, 22.06.2019 08:00, flakko1899
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1
You know the right answer?
An element x has a dibrom ide with the empirical formula xbr2 and a dichloride with the empirical fo...
Questions in other subjects:




Mathematics, 29.01.2020 01:56


Mathematics, 29.01.2020 01:56

Mathematics, 29.01.2020 01:56


Mathematics, 29.01.2020 01:56


 mol
mol gives 1 mole of
gives 1 mole of  . then
. then 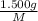 mol of [tex[CBr_2[/tex] will give:
mol of [tex[CBr_2[/tex] will give: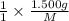 mol=\frac{1.500 g}{M}[/tex] mol[/tex] of
mol=\frac{1.500 g}{M}[/tex] mol[/tex] of