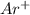Chemistry, 15.01.2020 19:31 rosaliaempress8377
An atomic cation with a charge of +1 has the following electron configuration: 1s^2 2s^2 2p^6 3s^2 3p^5 what is the chemical symbol for the ion? how many electrons does the ion have? how many 3d electrons are in the ion?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, brookekolmetz
How many orbitals does the p sub shell container
Answers: 3

Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 13:30, annanikherrera
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3

Chemistry, 22.06.2019 15:30, dylannhandy
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2
You know the right answer?
An atomic cation with a charge of +1 has the following electron configuration: 1s^2 2s^2 2p^6 3s^2...
Questions in other subjects:





Computers and Technology, 12.03.2020 20:29

Mathematics, 12.03.2020 20:29