Chemistry, 15.01.2020 06:31 santiagobermeo32
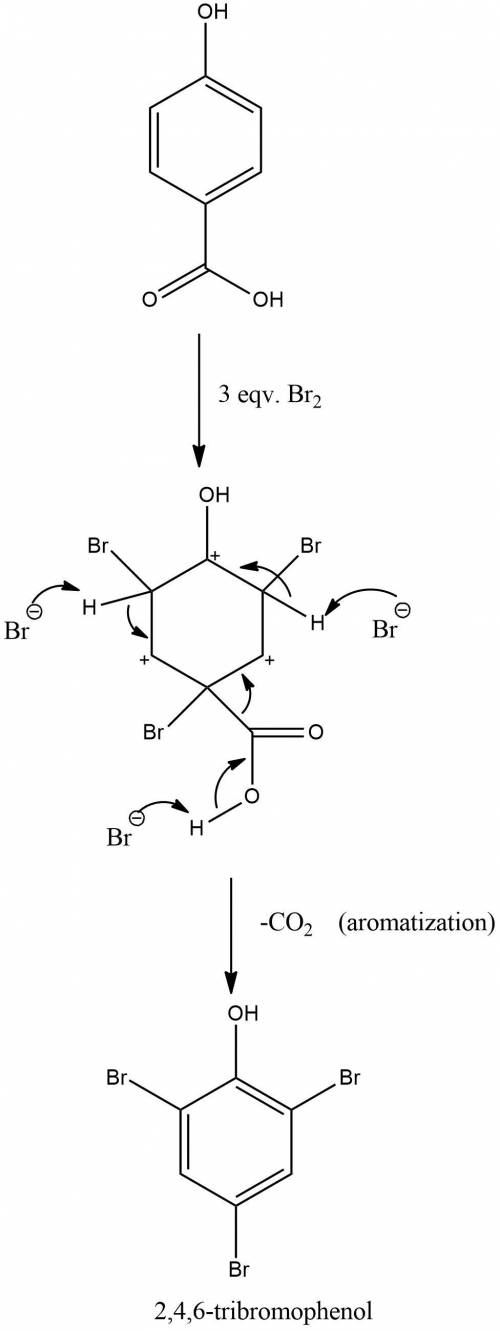
Treatment of p-hydroxybenzoic acid with aqueous bromine leads to the evolution of carbon dioxide and the formation of 2,4,6-tribromophenol. explain.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:50, Catracho3619
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 22.06.2019 06:00, girly37
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3


Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
You know the right answer?
Treatment of p-hydroxybenzoic acid with aqueous bromine leads to the evolution of carbon dioxide and...
Questions in other subjects:


History, 20.11.2020 21:30


Chemistry, 20.11.2020 21:30




Mathematics, 20.11.2020 21:30