Chemistry, 15.01.2020 06:31 josiahsurfer
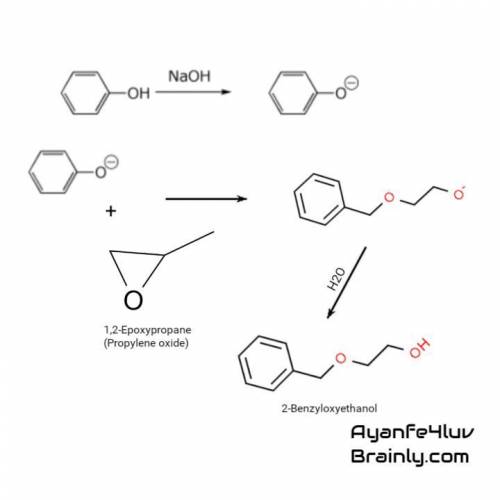
Reaction of phenol with 1,2-epoxypropane in aqueous sodium hydroxide at 150°c gives a single product, c9h12o2. in 90% yield. suggest a reasonable structure for this compound.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 22:00, huddyxo
Scientists often have to deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each number in an alternate form.
Answers: 1

Chemistry, 23.06.2019 02:20, alejandraluna95
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1

Chemistry, 23.06.2019 07:30, jessicawelch25
In a laboratory determination of the atomic weight of tin, a sample of tin is weighed in a crucible. nitric acid is added, and the reaction proceeds to give a hydrated tin(iv)oxide plus no2and h2o. the hydrated tin(iv)oxide is then heated strongly and reacts as follows: sno2.xh2o(s)sno2(s)+ xh2o(g)the sno2is finally cooled and weighed in the crucible. explain the effect on the calculated atomic weight of tin that would result from each of the following experimental errors: (a)considerable spattering occurs when the nitric acid is added to the tin.(b)the hydrated tin(iv)oxide is not heated sufficiently to change it completely to tin oxide.
Answers: 2
You know the right answer?
Reaction of phenol with 1,2-epoxypropane in aqueous sodium hydroxide at 150°c gives a single product...
Questions in other subjects:

Mathematics, 23.11.2019 02:31


Mathematics, 23.11.2019 02:31


History, 23.11.2019 02:31

Biology, 23.11.2019 02:31

Biology, 23.11.2019 02:31


Physics, 23.11.2019 02:31