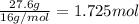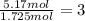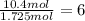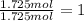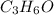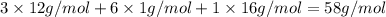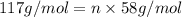Chemistry, 15.01.2020 04:31 alondrasanchezvillan
Compound x, isolated from lanolin (sheep's wool fat), has the pungent aroma of dirty sweatsocks. a careful analysis showed that compound x contains 62% carbon and 10.4% hydrogen. no nitrogen or halogen was found.
a. compute an empirical formula for compound x.
express your answer as a condensed structural formula.
b. a molecular weight determination showed that compound x has a molecular weight of approximately 117. find the molecular formula of compound x.
express your answer as a condensed structural formula.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, PrincessKeliah5538
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1


Chemistry, 22.06.2019 21:00, ciel8809
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1

Chemistry, 22.06.2019 22:00, aliciaa101
Ill give u brainliest pls how is mass of carbon conserved during cellular respiration
Answers: 1
You know the right answer?
Compound x, isolated from lanolin (sheep's wool fat), has the pungent aroma of dirty sweatsocks. a c...
Questions in other subjects:

Mathematics, 11.11.2020 07:50


Mathematics, 11.11.2020 07:50

History, 11.11.2020 07:50

Mathematics, 11.11.2020 07:50





Mathematics, 11.11.2020 07:50

 .
.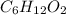 .
.