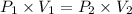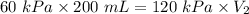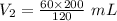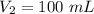The pressure on a 200-milliliter sample of co2(g) at
constant temperature is increased from 60...

Chemistry, 15.01.2020 04:31 moonlighthowl4537
The pressure on a 200-milliliter sample of co2(g) at
constant temperature is increased from 60 kpa to
120 kpa. what is the new volume of the gas? a) 100 ml b) 300 ml
c) 400 ml d) 600 ml

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, bakoeboo
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2



Chemistry, 22.06.2019 08:30, Apple557
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1
You know the right answer?
Questions in other subjects:



Mathematics, 02.02.2021 06:20

Spanish, 02.02.2021 06:20

History, 02.02.2021 06:20

Mathematics, 02.02.2021 06:20

Mathematics, 02.02.2021 06:20



Mathematics, 02.02.2021 06:30