Chemistry, 14.01.2020 23:31 morenodonaldo762
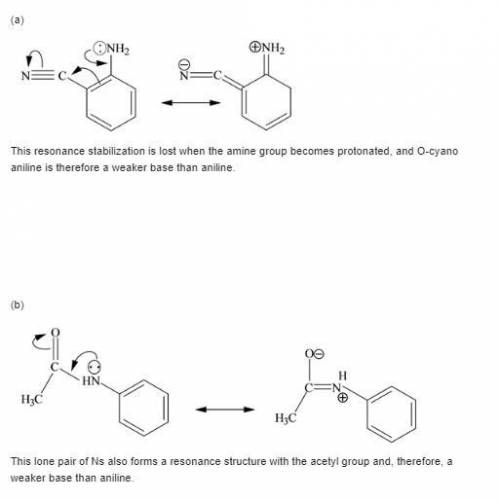
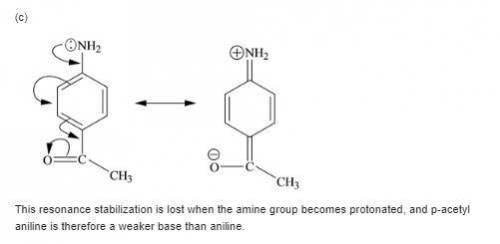
Each of the following is a much weaker base than aniline. present a resonance argument to explain the effect of the substituent in each case.
a. o-cyanoaniiine
b. p-aminoacetophenone
c. c6h5nhcch3
||
o

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2


Chemistry, 23.06.2019 01:00, shartiarahoward
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3
You know the right answer?
Each of the following is a much weaker base than aniline. present a resonance argument to explain th...
Questions in other subjects:




Mathematics, 15.10.2019 19:00

History, 15.10.2019 19:00