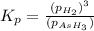Chemistry, 14.01.2020 23:31 coochieboi
The gas arsine, ash3, decomposes as follows. 2 ash3(g) equilibrium reaction arrow 2 as(s) + 3 h2(g) in an experiment at a certain temperature, pure ash3(g) was placed in an empty, rigid, sealed flask at a pressure of 392.0 torr. after 48 hours the pressure in the flask was observed to be constant at 488.0 torr. (a) calculate the equilibrium pressure of h2(g).

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 14:00, hammackkatelyn60
The content of manganese (mn) in steel was determined spectrophotometrically and with the use of the standard addition method. an unknown sample of mn from a digested steel sample gave an absorbance of 0.185 when analyzed spectrophotometrically. when 5.00 ml of solution containing 95.5 ppm mn was added to 50.0 ml of the unknown steel solution (digested sample), the absorbance was 0.248. calculate the concentration, in parts-per-million (ppm), of mn in the digested steel sample solution.
Answers: 3

Chemistry, 23.06.2019 00:00, maronetham6253
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 01:00, liamgreene90
The time that is taken by neptune once around the sun is called
Answers: 1
You know the right answer?
The gas arsine, ash3, decomposes as follows. 2 ash3(g) equilibrium reaction arrow 2 as(s) + 3 h2(g)...
Questions in other subjects:




Biology, 14.11.2020 22:40

History, 14.11.2020 22:40

English, 14.11.2020 22:40


History, 14.11.2020 22:40

Chemistry, 14.11.2020 22:40

Mathematics, 14.11.2020 22:40

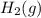 is, 288 torr
is, 288 torr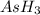 = 392.0 torr
= 392.0 torr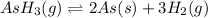
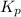 for the reaction will be:
for the reaction will be: