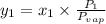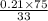Chemistry, 14.01.2020 20:31 danielamejia13
At 20 °c, the vapor pressure of benzene(c6h8) is 75 torr, and that of toluene(c7h8) is 22 torr. assuming that benzene and toluene from and idea solution.
a) what is the composition in mole fractions of a solution that has a vapor pressure of 33 torr at 20 °c
b) what is the mole fraction of benzene in the vapor above the solution described in part a?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:10, rightstrong9827
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3



Chemistry, 22.06.2019 15:20, shanyeah
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1
You know the right answer?
At 20 °c, the vapor pressure of benzene(c6h8) is 75 torr, and that of toluene(c7h8) is 22 torr. assu...
Questions in other subjects:


Mathematics, 07.11.2019 13:31

Biology, 07.11.2019 13:31


History, 07.11.2019 13:31

Mathematics, 07.11.2019 13:31



Biology, 07.11.2019 13:31

Chemistry, 07.11.2019 13:31

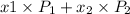
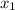 = mole fraction of component one
= mole fraction of component one
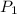 = vapor pressure of component one when pure
= vapor pressure of component one when pure
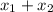 = 1
= 1
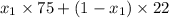
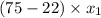
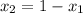
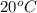 is 0.79.
is 0.79.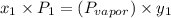
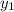 = composition in gas phase
= composition in gas phase