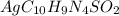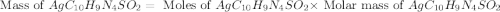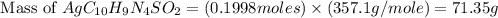Chemistry, 14.01.2020 01:31 xxaurorabluexx
Silver sulfadiazine burn-treating cream creates a barrier against bacterial invasion and releases antimicrobial agents directly into the wound. if 25.0 g of ag2o is reacted with 50.0 g of c10h10n4so2, what mass of silver sulfadiazine (agc10h9n4so2) can be produced, assuming 100% yield? ag2o(s)+2c10h10n4so2(s)⟶2agc10h9n4s o2(s)+h2o(l)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:50, Jerrikasmith28
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 22:20, icantspeakengles
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 22.06.2019 23:00, SophieCasey
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
Silver sulfadiazine burn-treating cream creates a barrier against bacterial invasion and releases an...
Questions in other subjects:


Mathematics, 24.03.2021 19:20



English, 24.03.2021 19:20


Mathematics, 24.03.2021 19:20

Mathematics, 24.03.2021 19:20


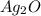 = 25.0 g
= 25.0 g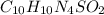 = 50.0 g
= 50.0 g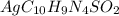 = 357.1 g/mole
= 357.1 g/mole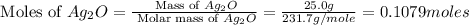
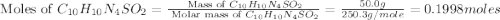
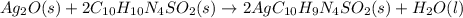
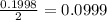 moles of
moles of