rate = k[n_2]^3 [o_3]

Chemistry, 14.01.2020 00:31 helphelp81
The rate of a certain reaction is given by the following rate law:
rate = k[n_2]^3 [o_3]
use this information to answer the questions below.
1. what is the reaction order in n_2?
2. what is the reaction order in o_3?
3. what is overall reaction order?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, emilyborland50
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d. the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 02:10, apowers6361
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 20:10, sarahalexa19
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 20:30, trevorhenyan51
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
The rate of a certain reaction is given by the following rate law:
rate = k[n_2]^3 [o_3]
rate = k[n_2]^3 [o_3]
Questions in other subjects:




Physics, 29.08.2019 10:30





Mathematics, 29.08.2019 10:30

Physics, 29.08.2019 10:30

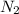 = 3
= 3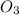 = 1
= 1![rate = k[N_2]^3 [O_3]](/tpl/images/0453/5258/fc831.png)


