Chemistry, 13.01.2020 19:31 nayelimoormann
A0.483-g sample of nonanedioic acid (c9h16o4) is burned in a bomb calorimeter and the temperature increases from 24.70 °c to 27.20 °c. the calorimeter contains 1.01×103 g water and the bomb has a heat capacity of 867 j/°c. based on this experiment, calculate δe (kj/mol) for the combustion reaction.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, LarryJoeseph
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 03:00, HHHHHHHHHMMMMMMMMM
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1


Chemistry, 22.06.2019 16:00, matt16913
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
A0.483-g sample of nonanedioic acid (c9h16o4) is burned in a bomb calorimeter and the temperature in...
Questions in other subjects:


Mathematics, 11.05.2021 05:10

Business, 11.05.2021 05:10

English, 11.05.2021 05:10


Mathematics, 11.05.2021 05:10


Mathematics, 11.05.2021 05:10

Mathematics, 11.05.2021 05:10

![q=[q_1+q_2]](/tpl/images/0453/1616/341bc.png)
![-q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0453/1616/37582.png)
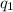 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter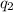 = heat absorbed by the water
= heat absorbed by the water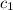 = specific heat of calorimeter =
= specific heat of calorimeter = 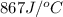
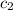 = specific heat of water =
= specific heat of water = 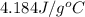
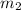 = mass of water =
= mass of water = 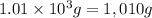
 = change in temperature = 27.20 °C - 24.70 °C =2.5°C
= change in temperature = 27.20 °C - 24.70 °C =2.5°C![-q=[(867 J/^oC\times 2.5 ^oC)+(1,010\times 4.184J/g^oC\times 2.5^oC)]](/tpl/images/0453/1616/a0608.png)
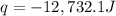
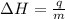
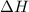 = enthalpy change = ?
= enthalpy change = ?