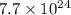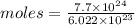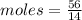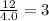Chemistry, 13.01.2020 09:31 leopolesamoy
Aparticular compound in the chemistry laboratory is found to contain 7.2x10^24 atoms of oxygen, 56.0g of nitrogen, and 4.0 mol of hydrogen. what is it’s empirical formula?


Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:00, deaishaajennings123
What is the equilibrium constant of aa+bb=cc+dd
Answers: 1

Chemistry, 22.06.2019 02:40, hardwick744
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2

Chemistry, 22.06.2019 14:30, emilymartinez75
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
Aparticular compound in the chemistry laboratory is found to contain 7.2x10^24 atoms of oxygen, 56.0...
Questions in other subjects:



Mathematics, 22.08.2019 09:30


Mathematics, 22.08.2019 09:30

English, 22.08.2019 09:50


History, 22.08.2019 09:50


Mathematics, 22.08.2019 09:50

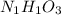
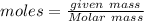
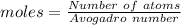
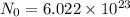 atoms
atoms