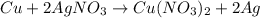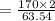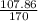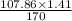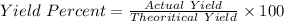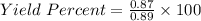Chemistry, 13.01.2020 05:31 davelopez979
In the copper – silver nitrate lab copper medals and silver nitrate solution reacted to produce silver metal and copper (ii) nitrate in solution. a student placed a copper wire with a mass of 2.93 g in the reaction test tube. the silver nitrate solution contained 1.41 g of silver nitrate. he obtained .87 g of silver metal. calculate the percent yield of silver.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:00, heggestade
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3


Chemistry, 22.06.2019 22:40, destineysarah
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
In the copper – silver nitrate lab copper medals and silver nitrate solution reacted to produce silv...
Questions in other subjects:




History, 16.12.2019 23:31



History, 16.12.2019 23:31


Chemistry, 16.12.2019 23:31

English, 16.12.2019 23:31