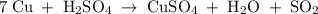Chemistry, 13.01.2020 01:31 rodfam13716
7. copper + sulfuric acid yield copper (ii) sulfate + water + sulfur dioxide

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3


Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
7. copper + sulfuric acid yield copper (ii) sulfate + water + sulfur dioxide...
Questions in other subjects:


English, 16.09.2021 23:40

Chemistry, 16.09.2021 23:40

Mathematics, 16.09.2021 23:40


Mathematics, 16.09.2021 23:40

Biology, 16.09.2021 23:40

Social Studies, 16.09.2021 23:40

Mathematics, 16.09.2021 23:40

Mathematics, 16.09.2021 23:40