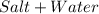Chemistry, 13.01.2020 01:31 weirdojuwin
Neutralization, the chemical reaction combining an acid with a base to produce a neutral solution, produces a salt and
water.
hydrogen gas.
o
carbon dioxide
d)
metallic precipitates.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, monithebtslover01
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1


Chemistry, 22.06.2019 20:00, aksambo4707
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

You know the right answer?
Neutralization, the chemical reaction combining an acid with a base to produce a neutral solution, p...
Questions in other subjects:

Mathematics, 18.08.2021 05:20


Mathematics, 18.08.2021 05:20


Mathematics, 18.08.2021 05:20


Physics, 18.08.2021 05:20


Mathematics, 18.08.2021 05:20

Spanish, 18.08.2021 05:20

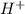 ) ion and negative non-metal ions. A base when dissolved in water produces positive metal ions and negative hydroxide (
) ion and negative non-metal ions. A base when dissolved in water produces positive metal ions and negative hydroxide (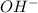 ) ions. When an acid reacts with a base, the hydrogen ions react with the hydroxide ions to form water.
) ions. When an acid reacts with a base, the hydrogen ions react with the hydroxide ions to form water.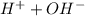 →
→ 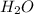
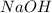 ) and hydrochloric acid (
) and hydrochloric acid (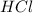 ) forms Water (
) forms Water (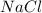 )
)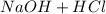 ⇄
⇄ 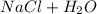
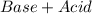 ⇄
⇄