
Calculate the voltage of the cell.
ag(s)|agbr(s), nabr(aq, 1.0 m)||cdcl2(aq, 0.050 m)|cd(s)
agbr(s) + e- ⇌ ag(s) + br-
eo = 0.071 v
ag+ + e- ⇌ ag(s)
eo = 0.799 v
cd2+ + 2e- ⇌ cd(s)
eo = -0.402 v
ksp (agbr(s)) = 5.0 × 10-13
a. -0.678 v
b. -0.511 v
c. -0.423 v
d. 0.511 v

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 20:30, huangjianhe135
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3


Chemistry, 23.06.2019 03:30, antoinetteee03
Name atleast 3 type of energy associated with the microwave
Answers: 1
You know the right answer?
Calculate the voltage of the cell.
ag(s)|agbr(s), nabr(aq, 1.0 m)||cdcl2(aq, 0.050 m)|cd...
ag(s)|agbr(s), nabr(aq, 1.0 m)||cdcl2(aq, 0.050 m)|cd...
Questions in other subjects:


English, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01


Mathematics, 16.10.2020 15:01


Biology, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01

Mathematics, 16.10.2020 15:01

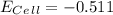
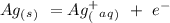 Eo = -0.799 V
Eo = -0.799 V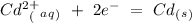 Eo = -0.402 V
Eo = -0.402 V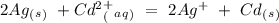 Eo = -1.201
Eo = -1.201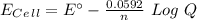
![E_C_e_l_l=E^{\circ}-\frac{0.0592}{n}Log\frac{[Ag^+]^2}{[Cd^2^+]}](/tpl/images/0450/4403/f186a.png)
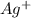 concentration, so:
concentration, so: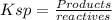
![5x10^-^1^3=[x][1]](/tpl/images/0450/4403/89725.png)
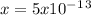
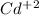 given by the problem we can calculate Q:
given by the problem we can calculate Q:![Q=\frac{[5x10^-^1^3]^2}{[0.05]}=5x10^-^2^4](/tpl/images/0450/4403/d0af4.png)
![E_C_e_l_l=-1.201}-\frac{0.0592}{2}Log[5x10^-^2^4]](/tpl/images/0450/4403/4601d.png)


