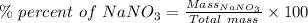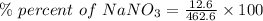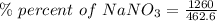Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, daniel9299
Consider the reaction: n2(g) + o2(g) ? 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3


Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1

Chemistry, 23.06.2019 13:30, reaunnatowns
These traits describe either a chemical or a nuclear reaction. which statements describe a nuclear reaction? check all that apply. involves the loss, gain, or sharing of electrons may involve a change in total mass involve relatively low energy changes occur outside the nucleus involve very high-energy changes involve changes in nuclides when decay occurs
Answers: 1
You know the right answer?
Calculate the mass percent solute in a solution of 12.6 grams of nano3 in 450 grams of water....
Questions in other subjects:


History, 03.03.2020 02:20








Biology, 03.03.2020 02:20

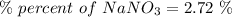
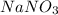 = 12.6 g
= 12.6 g