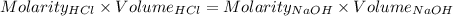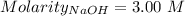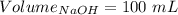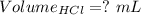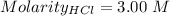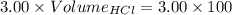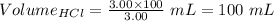Chemistry, 10.01.2020 02:31 coollid876
What volume of 3.00 m hcl will form a solution with a basic ph when mixed with 100 ml of 3.00 m naoh?
a)101 ml
b)100 ml
c)150 ml
d)50.0 ml

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

You know the right answer?
What volume of 3.00 m hcl will form a solution with a basic ph when mixed with 100 ml of 3.00 m naoh...
Questions in other subjects:










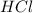 = Moles of NaOH
= Moles of NaOH