
Aluminum sulfate, known as cake alum, is used in dying leather and cloth and in purifying sewage. in basic solution, it reacts with hydroxide to form a white precipitate. what mass of precipitate forms when 163.2ml of .553 m sodium hydroxide is added to 627ml of a solution containing 0.0462 m aluminum sulfate. express answer in grams.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, uniqueray33
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1


Chemistry, 22.06.2019 14:20, greenbyron88
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 22:10, preachersgirl5
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Aluminum sulfate, known as cake alum, is used in dying leather and cloth and in purifying sewage. in...
Questions in other subjects:


Biology, 02.08.2019 15:30






Mathematics, 02.08.2019 15:30


Mathematics, 02.08.2019 15:30

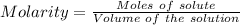
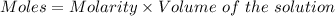
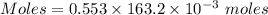
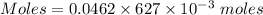
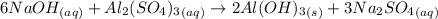
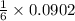 mole of aluminum sulfate
mole of aluminum sulfate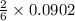 moles of the precipitate
moles of the precipitate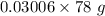 = 2.34468 g
= 2.34468 g

