
Chemistry, 09.01.2020 06:31 Gribblejames
A0.1276 g sample of an unknown monoprotic acid was dissolved in 25.0 ml of water and titrated with 0.0633 m naoh solution. the volume of base required to bring the solution to the equivalence point was 18.4 ml. (a) calculate the molar mass of the acid. (b) after 10.0 ml of base had been added during the titration, the ph was determined to be 5.87. what is the ka of the unknown acid?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, fgcherubin
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1


Chemistry, 22.06.2019 22:00, cooljariel11
Give more examples of this type of heat transfer:
Answers: 1
You know the right answer?
A0.1276 g sample of an unknown monoprotic acid was dissolved in 25.0 ml of water and titrated with 0...
Questions in other subjects:

English, 07.09.2021 22:50


Mathematics, 07.09.2021 23:00

Business, 07.09.2021 23:00

Mathematics, 07.09.2021 23:00

History, 07.09.2021 23:00



Mathematics, 07.09.2021 23:00

Mathematics, 07.09.2021 23:00

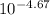 = 2.14 x 10⁻⁵
= 2.14 x 10⁻⁵

