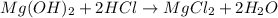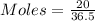Chemistry, 09.01.2020 06:31 deadpoolcorvettehats
In testing the effectiveness of an antacid compound, 20.0g of hydrochloric acid is mixed with 28.0g of magnesium hydroxide. will the base neutralize (completely use up) all of the acid? how much of which substance is in excess?
2hcl + (oh)2 -> mgcl2 + 2h20

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:00, youngchapo813p8d9u1
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
You know the right answer?
In testing the effectiveness of an antacid compound, 20.0g of hydrochloric acid is mixed with 28.0g...
Questions in other subjects:


English, 22.04.2020 00:41


Mathematics, 22.04.2020 00:41

History, 22.04.2020 00:41



Physics, 22.04.2020 00:41