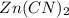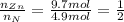Chemistry, 09.01.2020 03:31 twalters88
An unknown compound has the following chemical formula: zn(cn)x where x stands for a whole number. measurements also show that a certain sample of the unknown compound contains 9.7 mol of nitrogen and 4.9 mol of zinc. write the complete chemical formula for the unknown compound.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, clemsongirl5392
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 18:00, LuvieAnn1886
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3


Chemistry, 22.06.2019 22:10, preachersgirl5
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
An unknown compound has the following chemical formula: zn(cn)x where x stands for a whole number....
Questions in other subjects: