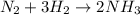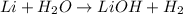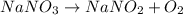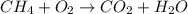2. classify each of the following reactions as a
synthesis, decomposition, single-displacement,...

2. classify each of the following reactions as a
synthesis, decomposition, single-displacement,
double-displacement, or combustion reaction:
a. n.(g) + 3h2(g) +2nh2(g)
b. 2li(s) + 2h,0(1) -2lioh(aq) + h2(g)
c. 2nano3(s) +2nano3(s) + 0,(9)
d. 2ch() + 190,(g) 1200,(g) + 14h, o(1)

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2


Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
Questions in other subjects:

Mathematics, 29.06.2019 19:00

Mathematics, 29.06.2019 19:00

English, 29.06.2019 19:00





Biology, 29.06.2019 19:00