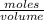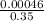Chemistry, 08.01.2020 02:31 bernicewhite156
Suppose you are a food chemist working for a company that makes and manufactures soda. your job is to create a new soft drink with the ingredients available to you. there is one problem—you need 1 liter of phosphoric acid solution, and the phosphoric acid available to use has a concentration of 0.01 m. this is much too high to add to your soda without distorting the flavor. typically, there are about 45 milligrams of phosphoric acid per 12 ounce can of soda. here is your task:
convert the 45 milligrams of phosphoric acid (h3po4) into grams and then into moles.
0.00046 moles or 4.6×10-4 moles
given that 12 ounces is equal to about 0.35 liter, find the molarity of the phosphoric acid in a typical can of soda (45 milligrams). 0.0013 m or 1.3×10-3 m
using the dilution equation, determine how much of the stock solution (0.01 m h3po4) you will need to make 1 liter of the concentration typically used in soda.
0.13 liter or 130 milliliters

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

You know the right answer?
Suppose you are a food chemist working for a company that makes and manufactures soda. your job is t...
Questions in other subjects:


Law, 15.10.2020 03:01


Computers and Technology, 15.10.2020 03:01

Mathematics, 15.10.2020 03:01

Mathematics, 15.10.2020 03:01

English, 15.10.2020 03:01