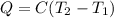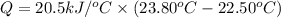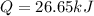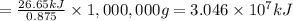A0.875-g sample of anthracite coal was burned in a bomb calorimeter. the temperature rose from 22.50 to 23.80°c. the heat capacity of the calorimeter was found in another experiment to be 20.5 kj/°c.
a. what was the heat evolved by the reaction?
b. what is the energy released on burning 1 metric ton (exactly 1000 kg) of this type of coal?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, carlinryan
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 14:10, cameronbeaugh
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 23.06.2019 06:00, womankrush538
Which of the following is a solution a- brewed coffee b-tomato juice c- ranch salad dressing d- muddy water
Answers: 1
You know the right answer?
A0.875-g sample of anthracite coal was burned in a bomb calorimeter. the temperature rose from 22.50...
Questions in other subjects:




Mathematics, 31.03.2021 01:10

English, 31.03.2021 01:10


Mathematics, 31.03.2021 01:10


Mathematics, 31.03.2021 01:10

 is the energy released on burning 1 metric ton of this type of coal
is the energy released on burning 1 metric ton of this type of coal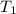 = 22.50°C
= 22.50°C = 23.80°C
= 23.80°C