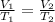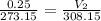Chemistry, 06.01.2020 18:31 allycoops666666
Acylinder with a movable piston contains a fixed amount of gas at a constant pressure. initially, the cylinder contains 0.25 liters of air at 0 degrees celsius. when the temperature is increased to 35 degrees celsius, the air will occupy what volume?
a. 0.28 l
b. 0.88 l
c. 8.75 l
d. 35.25 l

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:02, Julianhooks
What are the negative and positive impacts to society of making and using plastic bags?
Answers: 1

Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 23.06.2019 00:30, zaniathomasel
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
Acylinder with a movable piston contains a fixed amount of gas at a constant pressure. initially, th...
Questions in other subjects:

Mathematics, 26.08.2019 07:30


Mathematics, 26.08.2019 07:30

History, 26.08.2019 07:30

Social Studies, 26.08.2019 07:30

History, 26.08.2019 07:30

Social Studies, 26.08.2019 07:30



Health, 26.08.2019 07:30