
Chemistry, 06.01.2020 04:31 Silkyruthie
Consider the half reactions below for a chemical reaction.
> zn²⁺(aq)+2e⁻
cu²⁺(aq)+2e⁻> cu(s)
what is the overall equation for this chemical reaction?
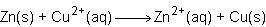

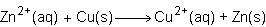
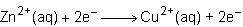

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2


Chemistry, 23.06.2019 11:00, landon6663
Which of the following reactions represents an exothermic reaction? nh3(g) + 12.0 kcal ½n2(g) + 3/2 h2(g) ch4 + 2o2 co2 + 2h2o + 212,800 cal c + 2s cs2, h = 27,550 cal c(graphite) c(diamond), h = 0.45 kcal 2h2o 2h2 + o2, h = +58 kcal
Answers: 1
You know the right answer?
Consider the half reactions below for a chemical reaction.
> zn²⁺(aq)+2e⁻
cu²⁺(aq)+2e...
> zn²⁺(aq)+2e⁻
cu²⁺(aq)+2e...
Questions in other subjects:




English, 23.05.2020 02:58

Mathematics, 23.05.2020 02:58

Mathematics, 23.05.2020 02:58

Computers and Technology, 23.05.2020 02:58


Mathematics, 23.05.2020 02:58



