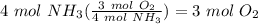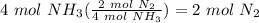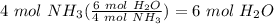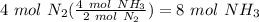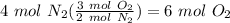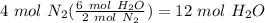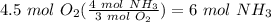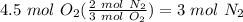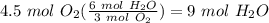Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 15:30, lizzyhearts
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3


Chemistry, 23.06.2019 01:00, bsheepicornozj0gc
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
Balance the following equation. then, given the moles of reactant or product below, determine the co...
Questions in other subjects:




Mathematics, 03.12.2020 01:00




Advanced Placement (AP), 03.12.2020 01:00

Chemistry, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00

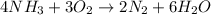 ". A complete explanation is below.
". A complete explanation is below.