
Chemistry, 03.01.2020 03:31 lucywood2024
Carbon monoxide at 25°c and steam at 150°c are fed to a continuous water-gas shift reactor. the product gas, which contains 50.0 mole% h2, 40.0% , and the balance emerges at 500°c at a rate of 2.50m3/h and goes to a condenser. the gas and liquid streams leaving the condenser are in equilibrium at 15°c and 1 bar. the liquid may be taken to be pure w"a) calculate the % excess steam fed to the reactor and rate of condensation of the water (kg/h) leaving the condensor. b) calculate the rate (kw) at which heat must be removed from the condensor. c) calculate the rate of heat transfer (kw) to or from the reactor (state which it is).

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 18:00, kingamir
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

Chemistry, 22.06.2019 21:00, agarcia24101993
What is the chemical formula for the compound formed between sodium and flour one
Answers: 1
You know the right answer?
Carbon monoxide at 25°c and steam at 150°c are fed to a continuous water-gas shift reactor. the prod...
Questions in other subjects:




Biology, 21.05.2020 12:57


History, 21.05.2020 12:57


Mathematics, 21.05.2020 12:57


 kW
kW
 = 40 moles
= 40 moles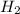 = 40 moles
= 40 moles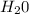 = 20 moles
= 20 moles
 %
%



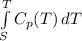
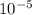 (-16324.231)
(-16324.231) (-16324.231) *
(-16324.231) *


