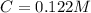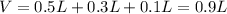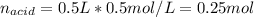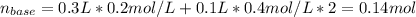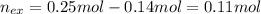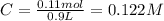Chemistry, 02.01.2020 23:31 PlzNoToxicBan
The following solutions are added together: .5l of .5m hcl, 300 ml of .2m naoh, and 100ml of .4m ca(oh)2. calculate the concentration of the excess acid or base

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, nayashuntel
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 23.06.2019 01:30, Sonicawesomeness
Select the correct answer from each drop-down menu. to make a table of the elements, dmitri mendeleev sorted the elements according to their . he then split the list of elements into several columns so that elements beside each other had similar .
Answers: 2

Chemistry, 23.06.2019 11:30, nickolasbradyp0hvwl
The density of e85 fuel is 0.801 g/ml. what is the mass of 1.00 gallon of the fuel? (1 gal. = 3.785 l)
Answers: 3
You know the right answer?
The following solutions are added together: .5l of .5m hcl, 300 ml of .2m naoh, and 100ml of .4m ca...
Questions in other subjects:

History, 03.09.2021 15:40

Biology, 03.09.2021 15:40



English, 03.09.2021 15:40





Mathematics, 03.09.2021 15:40