
Chemistry, 02.01.2020 23:31 kyleemarie2003
Which substance in each of the following pairs would you expect to have the higher boiling point? explain why.
a. ne or xe
b. co2 or cs2
c. ch4 or cl2
d. f2 or lif
e. nh3 or ph3

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, scottbrandon653
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1


Chemistry, 22.06.2019 20:00, bbyitskeke7160
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3
You know the right answer?
Which substance in each of the following pairs would you expect to have the higher boiling point? e...
Questions in other subjects:










History, 29.08.2020 02:01

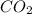 and
and 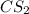 are non-polar in nature with intermolecular dispersion forces. So, more is the molecular weight of a compound more heat will be required by it in order to break the bonds.
are non-polar in nature with intermolecular dispersion forces. So, more is the molecular weight of a compound more heat will be required by it in order to break the bonds. 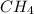 is 16 g/mol and molecular weight of
is 16 g/mol and molecular weight of 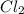 is 35 g/mol.
is 35 g/mol. 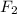 is a covalent compound so, it will also be non-polar in nature. Hence, it has weak intermolecular dispersion forces. On the other hand, LiF is an ionic compound and it has strong intermolecular forces of attraction due to the presence of opposite charges on the combining atoms.
is a covalent compound so, it will also be non-polar in nature. Hence, it has weak intermolecular dispersion forces. On the other hand, LiF is an ionic compound and it has strong intermolecular forces of attraction due to the presence of opposite charges on the combining atoms. 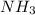 and
and 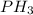 are polar molecules in which dipole-dipole forces does exist. Since, nitrogen atom is more electronegative than phosphorous atom so, it will have stronger hydrogen bonding and strong intermolecular forces.
are polar molecules in which dipole-dipole forces does exist. Since, nitrogen atom is more electronegative than phosphorous atom so, it will have stronger hydrogen bonding and strong intermolecular forces.

