
Calculate the standard entropy change for the industrial synthesis of urea (a common fertilizer): co2(g) + 2 nh3(g) → co(nh2)2(s) + h2o(ℓ) at 25◦c. patel (smp4358) – 28: fall exam review part three – lyssy – (112476) 8 s ◦ co2(g) 213.74 j k·mol nh3(g) 192.45 j k·mol co(nh2)2(s) 104.6 j k·mol h2o(ℓ) 69.91 j k·mol answer in units of j k · mol.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:50, ayoismeisjjjjuan
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x. xx ml
Answers: 1


Chemistry, 22.06.2019 17:30, shookiegriffin
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 23.06.2019 03:00, draveon6925
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
You know the right answer?
Calculate the standard entropy change for the industrial synthesis of urea (a common fertilizer): c...
Questions in other subjects:

Social Studies, 28.07.2019 13:00

History, 28.07.2019 13:00

History, 28.07.2019 13:00

Social Studies, 28.07.2019 13:00

Social Studies, 28.07.2019 13:00


Biology, 28.07.2019 13:00


Biology, 28.07.2019 13:00

Chemistry, 28.07.2019 13:00


 is:
is:
![\Delta S^o=[n_{CO(NH_2)_2}\times \Delta S^0_{(CO(NH_2)_2)}+n_{H_2O}\times \Delta S^0_{(H_2O)}]-[n_{CO_2}\times \Delta S^0_{(CO_2)}+n_{NH_3}\times \Delta S^0_{(NH_3)}]](/tpl/images/0438/1232/f3a9c.png)
 = entropy change of reaction = ?
= entropy change of reaction = ?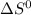 = standard entropy change
= standard entropy change = 104.6 J/mol.K
= 104.6 J/mol.K = 69.91 J/mol.K
= 69.91 J/mol.K = 213.74 J/mol.K
= 213.74 J/mol.K = 192.45 J/mol.K
= 192.45 J/mol.K![\Delta S^o=[1mole\times (104.6J/K.mole)+1mole\times (69.91J/K.mole)}]-[1mole\times (213.74J/K.mole)+2mole\times (192.45J/K.mole)]](/tpl/images/0438/1232/6f6bf.png)



