

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 17:00, smelcher3900
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 20:00, SpiritedAway7087
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3
You know the right answer?
Calculate the lattice energy of kcl(s) given the following data using the born-haber cycle: δhsubli...
Questions in other subjects:

Mathematics, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01

Biology, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01


Mathematics, 09.09.2020 22:01

Social Studies, 09.09.2020 22:01


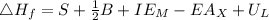
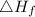 is enthalpy of formation
is enthalpy of formation 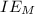 is ionisation enthalpy of metal
is ionisation enthalpy of metal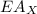 is electron affinity of non metal atom
is electron affinity of non metal atom 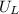 is lattice energy
is lattice energy 


