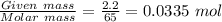Chemistry, 31.12.2019 05:31 carlosleblanc26
Zn + 2hcl → zncl2 + h2
at conditions of standard temperature and pressure, determine how many liters of hydrogen gas are produced by placing a zinc nail with a mass of 2.2g into an excess of hydrochloric acid.
a) 0.0015 l b) 0.75 l c) 1.33 l d) 6.42 l

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:40, georgehall3027
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 10:00, JOEFRESH10
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3
You know the right answer?
Zn + 2hcl → zncl2 + h2
at conditions of standard temperature and pressure, determine how many...
at conditions of standard temperature and pressure, determine how many...
Questions in other subjects:

Business, 15.12.2021 05:40


History, 15.12.2021 05:40

Mathematics, 15.12.2021 05:40

Mathematics, 15.12.2021 05:40


Mathematics, 15.12.2021 05:40


Mathematics, 15.12.2021 05:40