

Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1
You know the right answer?
1. the specific heat capacity of iron is 0.461 j g–1 k–1 and that of titanium is 0.544 j g–1 k–1. a...
Questions in other subjects:

Biology, 07.10.2021 16:50

Business, 07.10.2021 16:50

SAT, 07.10.2021 16:50

Business, 07.10.2021 16:50




Business, 07.10.2021 17:00

Health, 07.10.2021 17:00

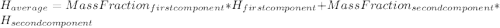
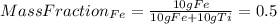
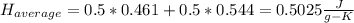
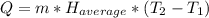
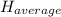 : The value obtained in equation (3)
: The value obtained in equation (3)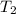 : Final temperature of the sample
: Final temperature of the sample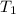 : Initial temperature of the sample
: Initial temperature of the sample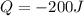 The mass of the whole sample is: 10g of Fe + 10g of Ti = 20g of sampleThe temperatures must be in absolute units of temperature (these are: rankine or kelvin)The initial temperature of the system is 100°C or 373K
The mass of the whole sample is: 10g of Fe + 10g of Ti = 20g of sampleThe temperatures must be in absolute units of temperature (these are: rankine or kelvin)The initial temperature of the system is 100°C or 373K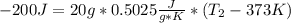
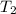
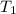 )
)

