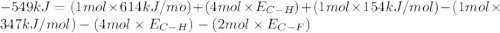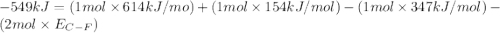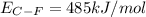Chemistry, 31.12.2019 02:31 yazanadel56
Consider the following reaction: c2h4(g) + f2(g) > c2h4f2(g) delta h = -549 kjestimate the carbon-fluorine bond energy given that the c-c bond energy is 347 kj/mol, the c=c bond energy is 614 kj/mol, and the f-f bond energy is 154 kj/mol.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 14:40, elawnnalewis4855
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1
You know the right answer?
Consider the following reaction: c2h4(g) + f2(g) > c2h4f2(g) delta h = -549 kjestimate the carbo...
Questions in other subjects:


Mathematics, 23.08.2019 13:00

Mathematics, 23.08.2019 13:00

Mathematics, 23.08.2019 13:00

English, 23.08.2019 13:00


Mathematics, 23.08.2019 13:00

Mathematics, 23.08.2019 13:00


![\Delta H_{rxn}=\sum [n_{i}\times (E_{bond})_{i}]-\sum [n_{j}\times (E_{bond})_{j}]](/tpl/images/0437/8404/d74f8.png)
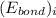 and
and 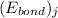 represents average bond energy in breaking "i" th bond and forming "j" th bond respectively.
represents average bond energy in breaking "i" th bond and forming "j" th bond respectively.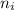 and
and 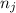 are number of moles of bond break and form respectively.
are number of moles of bond break and form respectively.