
Chemistry, 31.12.2019 01:31 icantspeakengles
Asolution of cacl 2 in water forms a mixture that is 42.0 % calcium chloride by mass. if the total mass of the mixture is 839.6 g, what masses of cacl 2 and water were used

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, KieraKimball
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1


Chemistry, 22.06.2019 22:30, eduardoguizar8787
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
Asolution of cacl 2 in water forms a mixture that is 42.0 % calcium chloride by mass. if the total m...
Questions in other subjects:


Mathematics, 02.09.2020 22:01

History, 02.09.2020 22:01

Mathematics, 02.09.2020 22:01

Geography, 02.09.2020 22:01


Mathematics, 02.09.2020 22:01

Mathematics, 02.09.2020 22:01

Mathematics, 02.09.2020 22:01

Mathematics, 02.09.2020 22:01

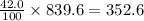 grams of calcium chloride
grams of calcium chloride

