Chemistry, 31.12.2019 00:31 katiems5514
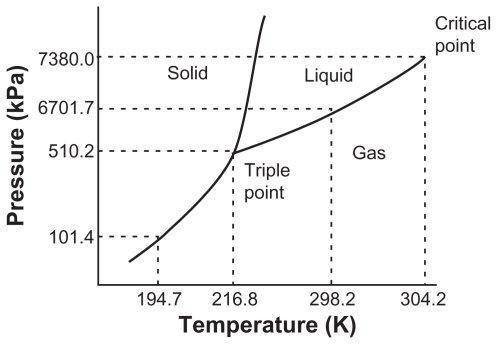
All three phases can exist in an equilibrium with one another at the __ of a substance

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1
You know the right answer?
All three phases can exist in an equilibrium with one another at the __ of a substance...
Questions in other subjects:


Mathematics, 06.05.2021 18:30

Law, 06.05.2021 18:30

Mathematics, 06.05.2021 18:30

English, 06.05.2021 18:30

Mathematics, 06.05.2021 18:30

History, 06.05.2021 18:30

Mathematics, 06.05.2021 18:30


Geography, 06.05.2021 18:30