
Chemistry, 30.12.2019 23:31 6224968918
Outline the steps needed to determine the limiting reactant when 30.0 g of propane, c3h8, is burned with 75.0 g of oxygen.
percent yield = 0.8347g / 0.9525 g × 100% = 87.6%

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, tiniecisneros28
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 22.06.2019 04:30, falishaduncanovmtz2
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1

Chemistry, 23.06.2019 03:00, makayyafreeman
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1
You know the right answer?
Outline the steps needed to determine the limiting reactant when 30.0 g of propane, c3h8, is burned...
Questions in other subjects:





Mathematics, 27.04.2021 20:50

Mathematics, 27.04.2021 20:50


English, 27.04.2021 20:50

Mathematics, 27.04.2021 20:50

SAT, 27.04.2021 20:50

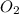
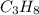 = 30.0 g
= 30.0 g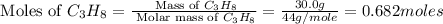
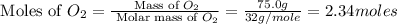
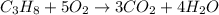
 moles of
moles of 

