
Astudent is doing a titration using potassium permanganate solution, kmno4, to determine the amount of h2o2 in a sample. the balanced equation for the reaction in the titration is given below:
2 mno4-(aq) + 6 h+(aq) + 5 h2o2(aq) --> ž 2 mn2+(aq) + 8 h2o(l) + 5 o2(g)
a student calculates an amount of moles of h2o2 that is larger than the actual value. which of the following errors could correctly explain the larger value?
the student failed to wear goggles.
the student did not swirl the flask appropriately and therefore stopped short of the endpoint.
the student failed to rinse the buret with kmno4¬ solution after rinsing it with distilled water.
the student added an extra 15 ml of distilled water to the h2o2 solution.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, 23gordns
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉mo re accurate estimates can be made with the van der waals equationí‘ťí‘ť=í‘›í‘›í‘›í‘›í‘›í‘›í‘ ‰í‘‰â’푛푛푟푟â’푞푞푛푛2í‘ ‰í‘‰2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1

Chemistry, 22.06.2019 09:30, psychocatgirl1
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone, due to its high light availability and warm water temperature
Answers: 3


Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3
You know the right answer?
Astudent is doing a titration using potassium permanganate solution, kmno4, to determine the amount...
Questions in other subjects:


English, 30.03.2021 22:30

Mathematics, 30.03.2021 22:30


Biology, 30.03.2021 22:30

Mathematics, 30.03.2021 22:30




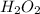 solution.
solution.

