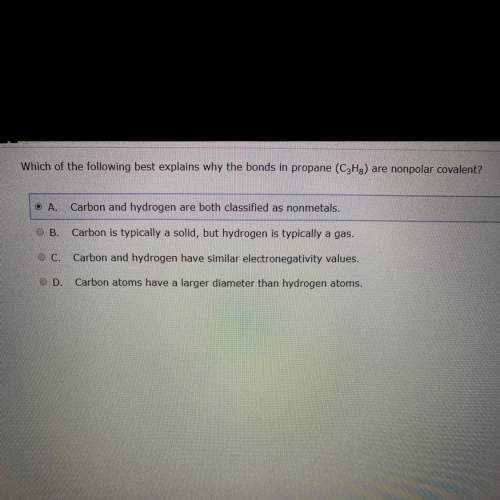
Which of the following best explains why the bond in propane (c3h8) are non polar covalent
...

Chemistry, 29.12.2019 23:31 cookiem0nster
Which of the following best explains why the bond in propane (c3h8) are non polar covalent

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, azzyla2003
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1



Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
Questions in other subjects:

Mathematics, 22.09.2019 16:20



Social Studies, 22.09.2019 16:20


Geography, 22.09.2019 16:20

Biology, 22.09.2019 16:20



Physics, 22.09.2019 16:20



