
At equilibrium, the concentrations of the products and reactants for the reaction, h2 (g) + i2 (g) 2 hi (g), are [h2] = 0.106 m; [i2] = 0.022 m; [hi] = 1.29 m calculate the new equilibrium concentration of hi (in m) if the equilibrium concentrations of h2 and i2 are 0.95 m and 0.019 m respectively.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, maddyjones4172
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3


Chemistry, 22.06.2019 21:00, ciel8809
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1
You know the right answer?
At equilibrium, the concentrations of the products and reactants for the reaction, h2 (g) + i2 (g) ...
Questions in other subjects:



Mathematics, 16.12.2019 05:31




Mathematics, 16.12.2019 05:31

Social Studies, 16.12.2019 05:31


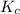 ) for the given chemical reaction, is given by the equation:
) for the given chemical reaction, is given by the equation:![K_{c} = \frac {[HI]^{2}}{[H_{2}]\: [I_{2}]}](/tpl/images/0435/6174/f8601.png)

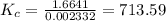
![K_{c} = \frac {[HI]^{2}}{(0.95\: M) \times (0.019\: M)}](/tpl/images/0435/6174/d2511.png)
![\Rightarrow K_{c} = 713.59 = \frac {[HI]^{2}}{0.01805}](/tpl/images/0435/6174/fef3c.png)
![\Rightarrow [HI]^{2} = 713.59 \times 0.01805 = 12.88](/tpl/images/0435/6174/d4ab1.png)
![\Rightarrow [HI] = \sqrt {12.88} = 3.589 M](/tpl/images/0435/6174/5120a.png)


