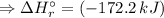Chemistry, 28.12.2019 05:31 lisamiller
Calculate horxn for the following reaction: h3aso4(aq) + 4 h2(g) --> ash3(g) + 4 h2o(l)(hof [ash3(g)] = 66.4 kj/mol; hof [h3aso4(aq)] = -904.6 kj/mol; hof [h2o(l)] = -285.8 kj/mol)

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 18:00, sandeebassett3
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2
You know the right answer?
Calculate horxn for the following reaction: h3aso4(aq) + 4 h2(g) --> ash3(g) + 4 h2o(l)(hof [a...
Questions in other subjects:




Mathematics, 28.05.2020 15:58




Biology, 28.05.2020 15:58

Physics, 28.05.2020 15:58

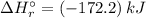
![\Delta H_{f}^{\circ } [H_{3}AsO_{4}(aq)]](/tpl/images/0435/4880/bc889.png) = -904.6 kJ/mol
= -904.6 kJ/mol![\Delta H_{f}^{\circ } [H_{2}(g)]](/tpl/images/0435/4880/b83d7.png) = 0 kJ/mol,
= 0 kJ/mol,![\Delta H_{f}^{\circ } [AsH_{3}(g)]](/tpl/images/0435/4880/eaaf4.png) = +66.4 kJ/mol
= +66.4 kJ/mol![\Delta H_{f}^{\circ } [H_{2}O(l)]](/tpl/images/0435/4880/46f61.png) = -285.8 kJ/mol
= -285.8 kJ/mol  = ?
= ?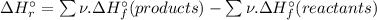
![\Delta H_{r}^{\circ } = [1 \times \Delta H_{f}^{\circ } [AsH_{3} (g)] + 4 \times \Delta H_{f}^{\circ } [H_{2}O(l)]] - [1 \times \Delta H_{f}^{\circ } [H_{3}AsO_{4}(aq)] + 4 \times \Delta H_{f}^{\circ } [H_{2}(g)]](/tpl/images/0435/4880/cee8f.png)
![\Rightarrow \Delta H_{r}^{\circ } = [1 \times (+66.4\,kJ/mol) + 4 \times (-285.8\,kJ/mol) ] - [1 \times (-904.6\,kJ/mol) + 4 \times (0\,kJ/mol)]](/tpl/images/0435/4880/49bdc.png)
![\Rightarrow \Delta H_{r}^{\circ } = [-1076.8\, kJ] - [-904.6\,kJ]](/tpl/images/0435/4880/a7183.png)