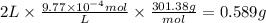Chemistry, 28.12.2019 01:31 ronniethefun
The drug dobutamine (fw=301.38g/mol, c18h23no3c18h23n03) has a molar absorptivity of 703 at 262nm. one tablet is dissolved in water and diluted to a volume of 2l. if the solution exhibits an absorbance of 0.687 in the uv region at 262nm in a 1- cm cell, how many grams to dobutamine are contained in the tablet? a. 0.5277g b. 0.58889 c. 9.77x 10g d. 97.778ge. 9.7778g

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bchagnard2122
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 13:00, torigirl4126
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 14:00, hannahhoskings6989
What was bohr’s contribution to the planetary model
Answers: 1

Chemistry, 22.06.2019 17:00, Estrella2209
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
The drug dobutamine (fw=301.38g/mol, c18h23no3c18h23n03) has a molar absorptivity of 703 at 262nm. o...
Questions in other subjects:

Mathematics, 25.06.2020 01:01


Mathematics, 25.06.2020 01:01

Spanish, 25.06.2020 01:01

Mathematics, 25.06.2020 01:01

Mathematics, 25.06.2020 01:01