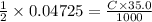We find that 18.90 milliliters of a 2.50m koh solution are required to titrate 35.0 milliters of a h2s04 solution. what is the molarity of the h2s04 solution? 2 2koh + h2s04 k2s04 2 h20 a. 0.675 m b. 1.57 m c. 0.0711 m d. 0.128 m e. 4.04 m f. 0.903 m g. 1.18 m h. 0.0173 m

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3


You know the right answer?
We find that 18.90 milliliters of a 2.50m koh solution are required to titrate 35.0 milliters of a h...
Questions in other subjects:




Mathematics, 16.02.2020 20:37

Mathematics, 16.02.2020 20:38

Biology, 16.02.2020 20:44

Mathematics, 16.02.2020 20:45



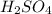 is 0.675 M
is 0.675 M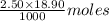 of KOH = 0.04725 moles of KOH
of KOH = 0.04725 moles of KOH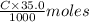 of
of