
Chemistry, 28.12.2019 00:31 quincyjosiah07
How much heat is absorbed/released when 20.00 g of nh3(g) reacts in the presence of excess o2 (g) to produce no (g) and h2o (l) according to the following chemical equation? 4nh3 (g) + 5o2 (g) > 4no (g) +6h2o (l) δ h: +1168 kja. 342.9 kj of heat are absorbed. b. 342.9 kj of heat are released. c. 1372 kj of heat are absorbed. d. 1372 kj of heat are released.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2


Chemistry, 22.06.2019 09:10, chloeholt123
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
How much heat is absorbed/released when 20.00 g of nh3(g) reacts in the presence of excess o2 (g) to...
Questions in other subjects:

Mathematics, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01

Social Studies, 06.10.2020 14:01



Mathematics, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01


Mathematics, 06.10.2020 14:01

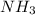 as:-
as:-
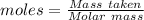
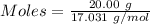
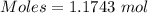
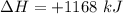
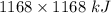 of heat
of heat

