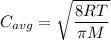Chemistry, 28.12.2019 00:31 GGerardi7552
A0.5 mol sample of he(g) and a 0.5 mol sample of ne(g) are placed separately in two 10.0 l rigid containers at 25°c. each container has a pinhole opening. which of the gases, he(g) or ne(g), will escape faster through the pinhole and why?
a) he because the he atoms are moving at a higher average speed than the ne atoms.
b) ne because its initial pressure in the container is higher
c) ne because the ne atoms have a higher average kinetic energy than the he atoms
d) both gases will escape at the same rate because the atoms of both gases have the same average kinetic enery

Answers: 3


Other questions on the subject: Chemistry




Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
A0.5 mol sample of he(g) and a 0.5 mol sample of ne(g) are placed separately in two 10.0 l rigid con...
Questions in other subjects:


Mathematics, 14.06.2021 01:50

Arts, 14.06.2021 01:50


Mathematics, 14.06.2021 01:50

Chemistry, 14.06.2021 01:50

Mathematics, 14.06.2021 01:50

History, 14.06.2021 01:50