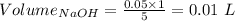Chemistry, 27.12.2019 05:31 svarner2001
Consider a solution that is 0.05 m hcl. your goal is to neutralize 1 l of this solution (i. e. bring the ph to 7). you also have a solution that is 5 m naoh. what volume of this solution should you add to the hcl solution, to neutralize it? provide your answer in units of liters (l).

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1


Chemistry, 22.06.2019 18:00, jalenclarke25
What volume would 2.25 moles of ne has occupy at stp?
Answers: 1
You know the right answer?
Consider a solution that is 0.05 m hcl. your goal is to neutralize 1 l of this solution (i. e. bring...
Questions in other subjects:



Mathematics, 26.01.2021 14:00

Mathematics, 26.01.2021 14:00

Mathematics, 26.01.2021 14:00



English, 26.01.2021 14:00

Business, 26.01.2021 14:00

History, 26.01.2021 14:00

 = Moles of NaOH
= Moles of NaOH